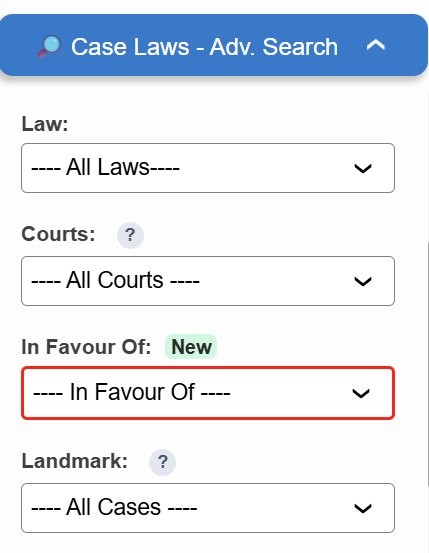
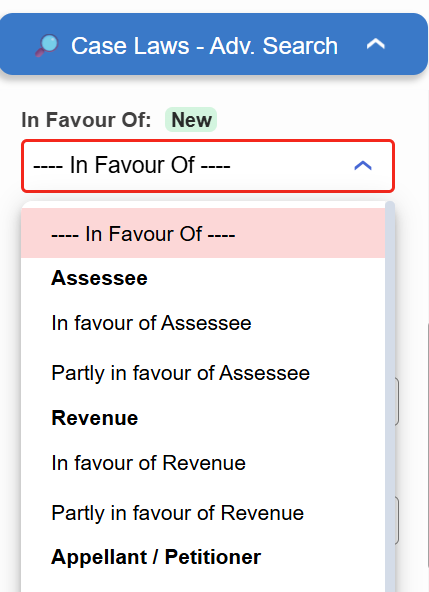
Introducing the “In Favour Of” filter in Case Laws.
- ⚖️ Instantly identify judgments decided in favour of the Assessee, Revenue, or Appellant
- 🔍 Narrow down results with higher precision
Try it now in Case Laws →
Just a moment...
Introducing the “In Favour Of” filter in Case Laws.
Try it now in Case Laws →


Press 'Enter' to add multiple search terms. Rules for Better Search
No Folders have been created
Are you sure you want to delete "My most important" ?
NOTE:
Don't have an account? Register Here
<h1>Govt allows Pharma Grade Sugar export under 25,000 MT cap with license and NABL test certificate required</h1> The government permits export of Pharma Grade Sugar under a restricted category with a total annual export cap of 25,000 MT. Exporters must hold a valid drug manufacturing license and submit NABL-accredited test certifications confirming compliance with Pharma Grade Sugar specifications at export. A one-time quota of 25,000 MT is allocated to bona fide pharmaceutical exporters for the current sugar season ending September 30, 2025. Applications are accepted online from June 20 to July 31, 2025, through the DGFT ECOM system, with only one application per IEC considered. Quotas will be allocated pro-rata based on production capacity, and authorizations remain valid for one year. Applicants must be registered members of the pharmaceutical export promotion council with a valid registration certificate. Late, incomplete, or non-compliant applications will be rejected. The DGFT reserves the right to modify allocation procedures as necessary.