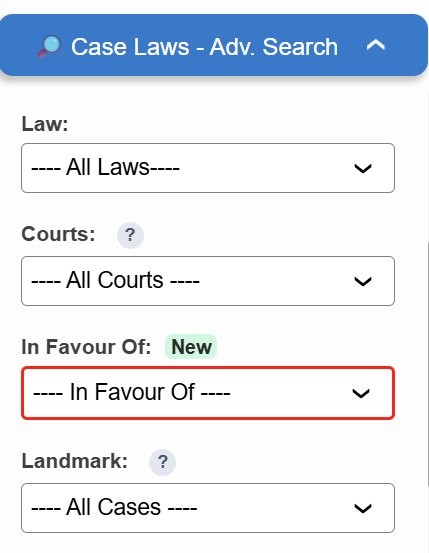
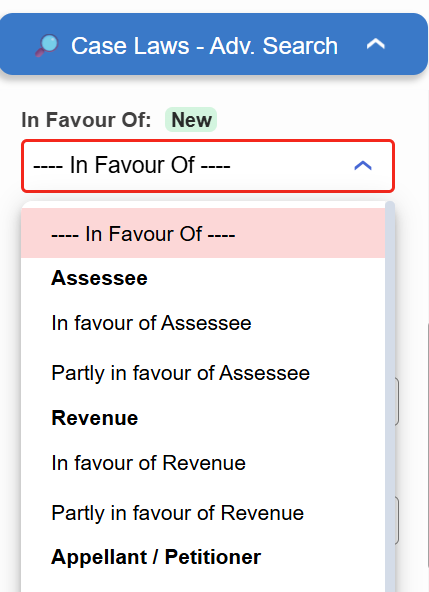
Introducing the “In Favour Of” filter in Case Laws.
- ⚖️ Instantly identify judgments decided in favour of the Assessee, Revenue, or Appellant
- 🔍 Narrow down results with higher precision
Try it now in Case Laws →
Just a moment...
Introducing the “In Favour Of” filter in Case Laws.
Try it now in Case Laws →


Press 'Enter' to add multiple search terms. Rules for Better Search
No Folders have been created
Are you sure you want to delete "My most important" ?
NOTE:
Don't have an account? Register Here
<h1>New Guidelines for Exporting Diagnostic Kits: Apply Online from August 5-8, 2020, with Required Documentation and Quotas.</h1> The Directorate General of Foreign Trade has issued guidelines for the export of diagnostic kits, including VTM, RNA Extraction, and RT-PCR kits, with specified export quotas for August. Only manufacturers can apply online for export authorizations, and applications must be submitted between August 5th and 8th, 2020. Eligibility requires proof of manufacturing, a single application per IEC, and specific documentation, including purchase orders and an undertaking of fulfilled domestic commitments. The export license will be valid for three months, and incomplete or late applications will not be considered. Approval is required from the Competent Authority.