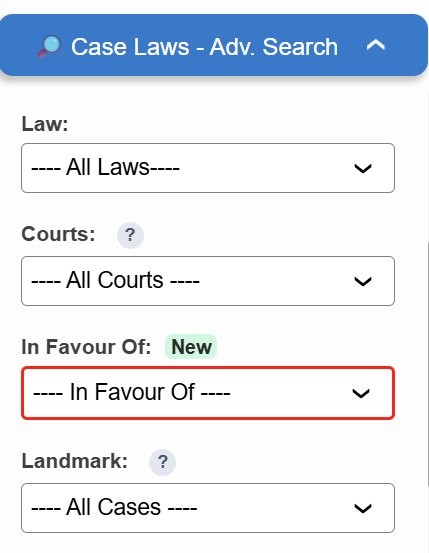
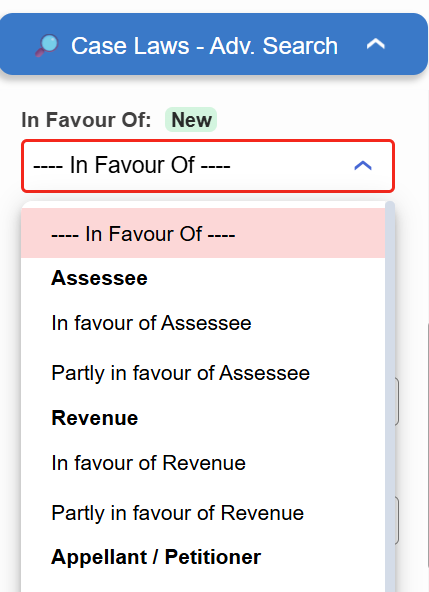
Introducing the “In Favour Of” filter in Case Laws.
- ⚖️ Instantly identify judgments decided in favour of the Assessee, Revenue, or Appellant
- 🔍 Narrow down results with higher precision
Try it now in Case Laws →
Just a moment...
Introducing the “In Favour Of” filter in Case Laws.
Try it now in Case Laws →


Press 'Enter' to add multiple search terms. Rules for Better Search
No Folders have been created
Are you sure you want to delete "My most important" ?
NOTE:
Don't have an account? Register Here
<h1>No Objection Certificate Not Needed for Drug Exports by Licensed Manufacturers, Simplifying India's Export Procedures.</h1> The circular issued by the Office of the Commissioner of Customs (Export) in New Delhi addresses the requirement of a 'No Objection Certificate' (NOC) from the Central Drug Standard Control Organization for exporting drugs. It states that an NOC will not be required if the shipping bills are filed by the manufacturer with a valid license under the Drugs and Cosmetics Act and Rules. This measure aims to simplify drug regulation practices in India concerning the export of drugs, medical devices, and cosmetics. Stakeholders must still adhere to the regulatory requirements of the importing countries. Any difficulties should be reported to the Commissioner.