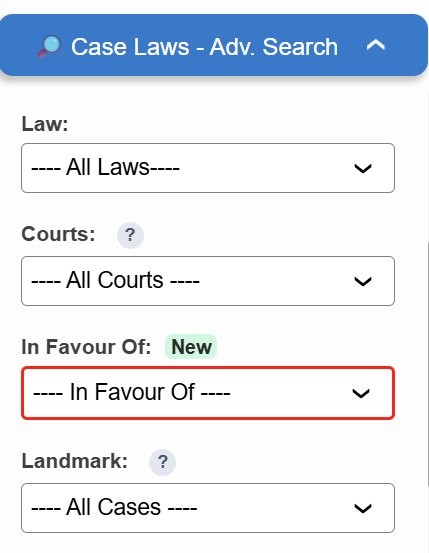
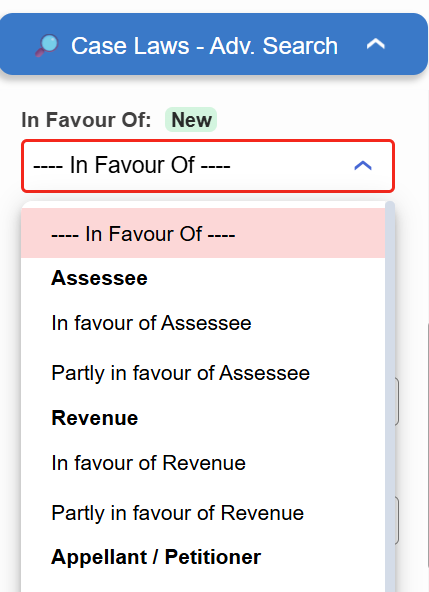
Introducing the “In Favour Of” filter in Case Laws.
- ⚖️ Instantly identify judgments decided in favour of the Assessee, Revenue, or Appellant
- 🔍 Narrow down results with higher precision
Try it now in Case Laws →
Just a moment...
Introducing the “In Favour Of” filter in Case Laws.
Try it now in Case Laws →


Press 'Enter' to add multiple search terms. Rules for Better Search
No Folders have been created
Are you sure you want to delete "My most important" ?
NOTE:
Don't have an account? Register Here
<h1>CDSCO Issues Guidelines for Re-importing Drugs and Cosmetics: Steps for ADC NOC and Quality Testing Explained.</h1> The circular outlines the procedure for re-importing drugs, cosmetics, and medicines as per the Central Drugs Standard Control Organization (CDSCO) guidelines. Importers, exporters, and customs house agents must follow specific steps to obtain the ADC No Objection Certificate (NOC). If drugs or cosmetics of Indian origin are re-imported, samples must be tested in government-approved laboratories. If found non-standard quality (NSQ), they may be released for reprocessing under supervision. The decision to release for reprocessing is made by the Deputy Drugs Controller, and the State Drug Controller or Zonal Officer must be informed. Stakeholders are advised to consult CDSCO documents for more details.