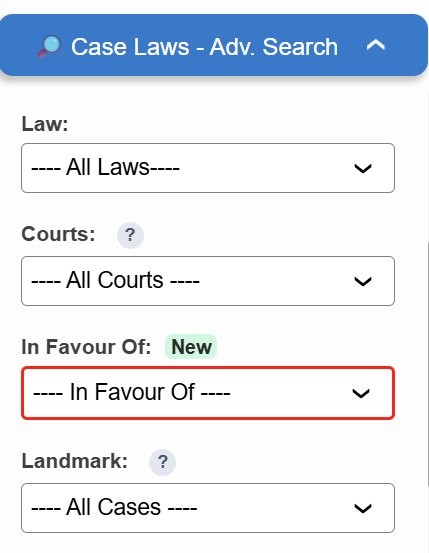
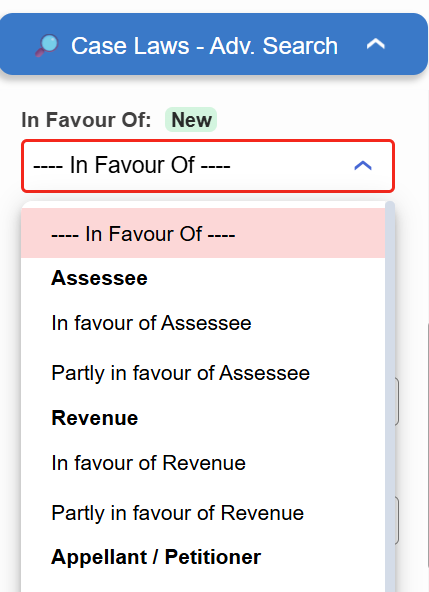
Introducing the “In Favour Of” filter in Case Laws.
- ⚖️ Instantly identify judgments decided in favour of the Assessee, Revenue, or Appellant
- 🔍 Narrow down results with higher precision
Try it now in Case Laws →
Just a moment...
Introducing the “In Favour Of” filter in Case Laws.
Try it now in Case Laws →


Press 'Enter' to add multiple search terms. Rules for Better Search
No Folders have been created
Are you sure you want to delete "My most important" ?
NOTE:
Don't have an account? Register Here
<h1>India Mandates Track and Trace System for Exported Pharmaceuticals; Barcoding Required at Multiple Packaging Levels.</h1> The circular from the Directorate General of Foreign Trade outlines the implementation of a Track and Trace system for exporting pharmaceuticals and drug consignments. Manufacturers and exporters must print barcodes at primary, secondary, and tertiary packaging levels following GS1 Global Standards. While primary level barcoding is temporarily exempt, details must be printed in human-readable form. The circular mandates data maintenance in a parent-child relationship for packaging levels, with specific exemptions for small-scale industries (SSI) until March 31, 2017. Data must be uploaded to a central portal before distribution. Exemptions apply for drugs manufactured before specified dates, and compliance with importing countries' requirements is considered.