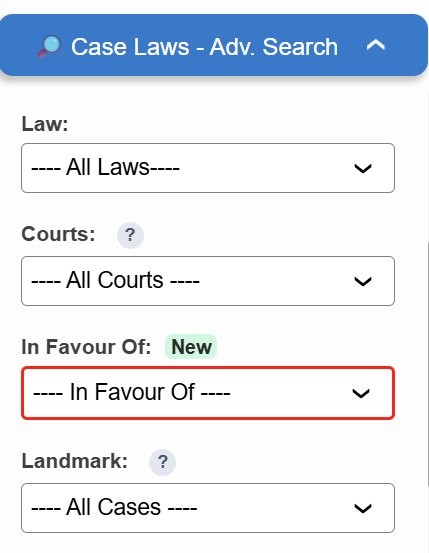
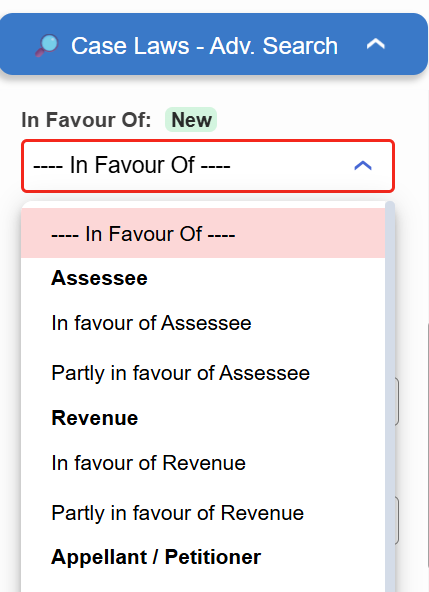
Introducing the “In Favour Of” filter in Case Laws.
- ⚖️ Instantly identify judgments decided in favour of the Assessee, Revenue, or Appellant
- 🔍 Narrow down results with higher precision
Try it now in Case Laws →
Just a moment...
Introducing the “In Favour Of” filter in Case Laws.
Try it now in Case Laws →


Press 'Enter' to add multiple search terms. Rules for Better Search
No Folders have been created
Are you sure you want to delete "My most important" ?
NOTE:
Don't have an account? Register Here
<h1>New Procedures for Tracking Pharmaceutical Exports: Barcode System Required; Primary Packaging Deferred; Exemptions Available.</h1> The Directorate General of Foreign Trade has established procedures for tracking and tracing pharmaceutical export consignments, superseding previous notices. Exporters must submit a Certificate of Analysis and adopt a barcode-based track and trace system at different packaging levels according to GS1 standards. Tertiary and Secondary Level packaging barcode requirements are already in effect, while Primary Level packaging requirements are deferred. Exporters may seek exemptions if importing countries have specific mandates. Manufacturers must maintain records for six months post-expiry, and a central tracking portal will be developed. Self-certification processes remain unchanged since May 2014.